
October 2024 | Present
As Founding Designer of Amazig, I co-created a digital platform to accelerate the go-to-market process for pharmaceuticals. The tool provides digital administrative tracking aligned with international regulatory standards, reducing delays and human error in a highly complex environment.
The Challenge
Bringing a new drug to market requires
years of administrative coordination across legal, regulatory, and
medical teams. Processes were fragmented, error-prone, and
inconsistent between countries. How might we standardize regulatory
tracking to reduce time-to-market? How might we ensure compliance by
design, without slowing down innovation?
My Role
Define product scope and functionality,
conduct benchmarks and discovery sessions with SMEs, design wireframes
and high-fidelity screens, and structure the Agile design process to
integrate tasks into the development workflow while balancing user
needs, technical feasibility, and business constraints.
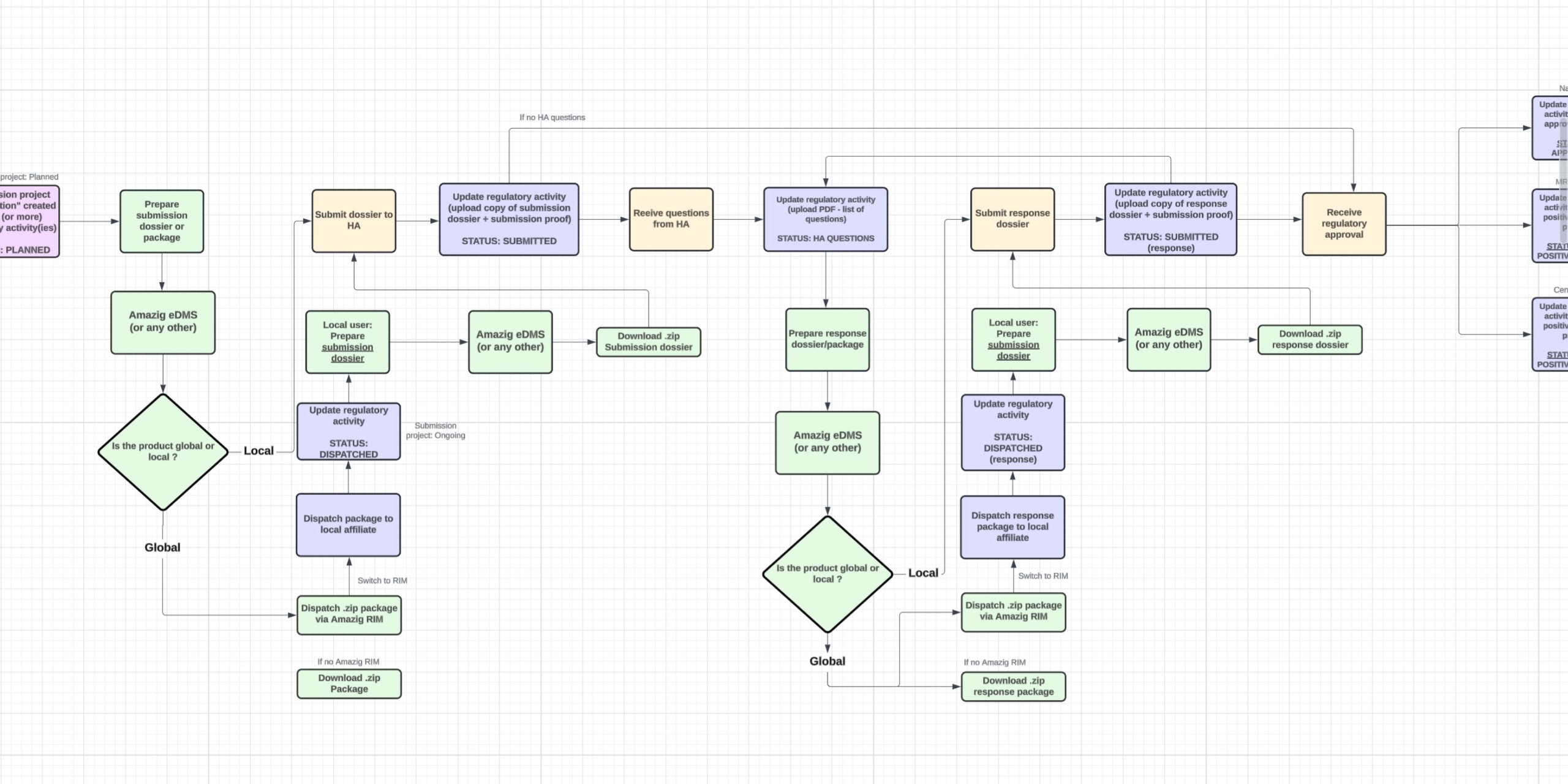
Solution
Create a unified digital platform for
regulatory tracking that streamlines processes across teams, minimizes
administrative delays, and reduces compliance mistakes. Ensure
alignment with international standards from the outset and present a
clickable prototype for validation with subject matter experts and
early users.
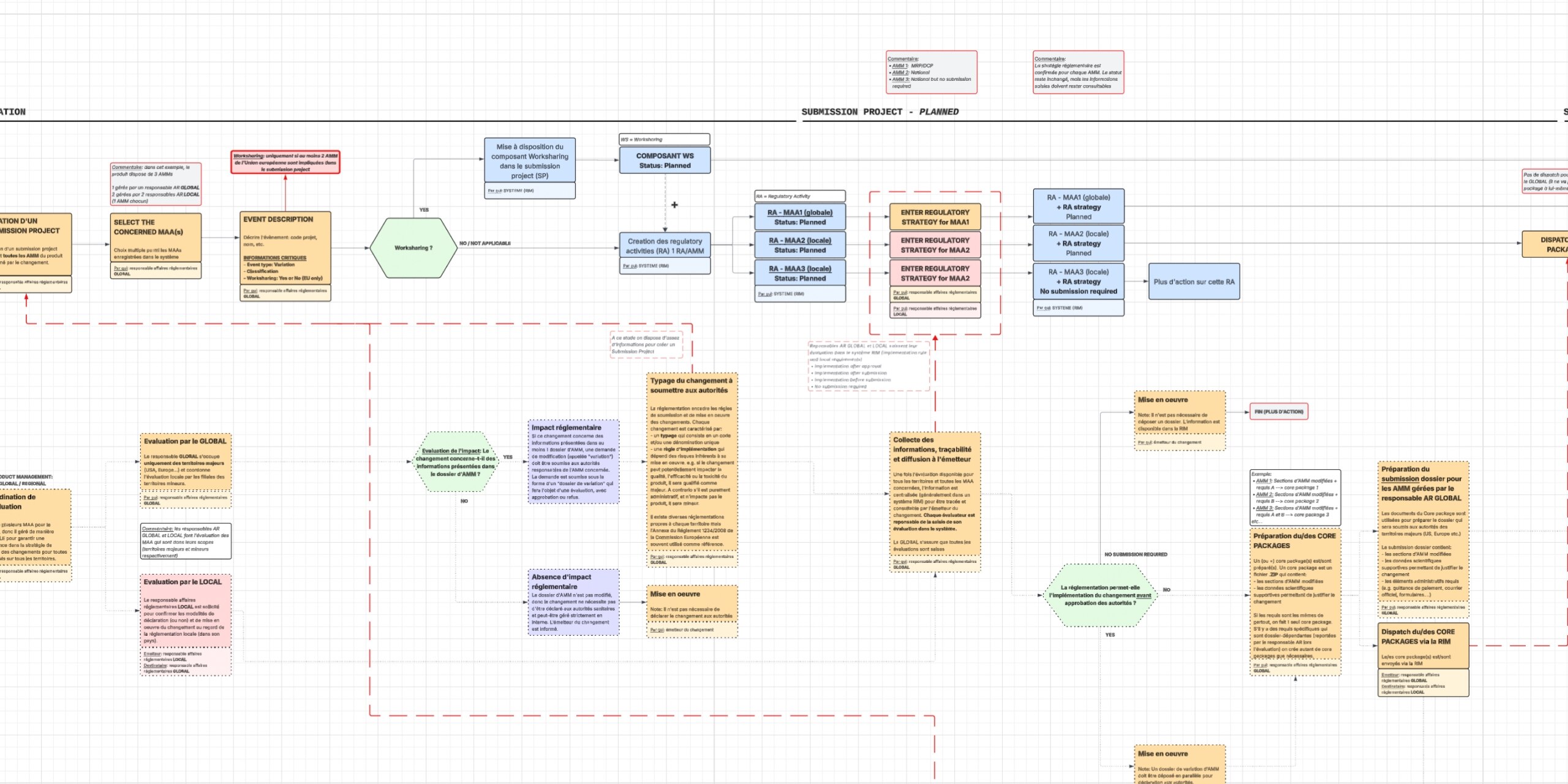
My Approach
Mapping administrative workflows and
compliance dependencies while wireframing essential flows like project
creation, submission, validation, and reporting. Quick feedback cycles
with SMEs help me validate feasibility. Prototyping the most important
user journeys and building a solid system foundation with consistent
components and modular layouts for future growth.
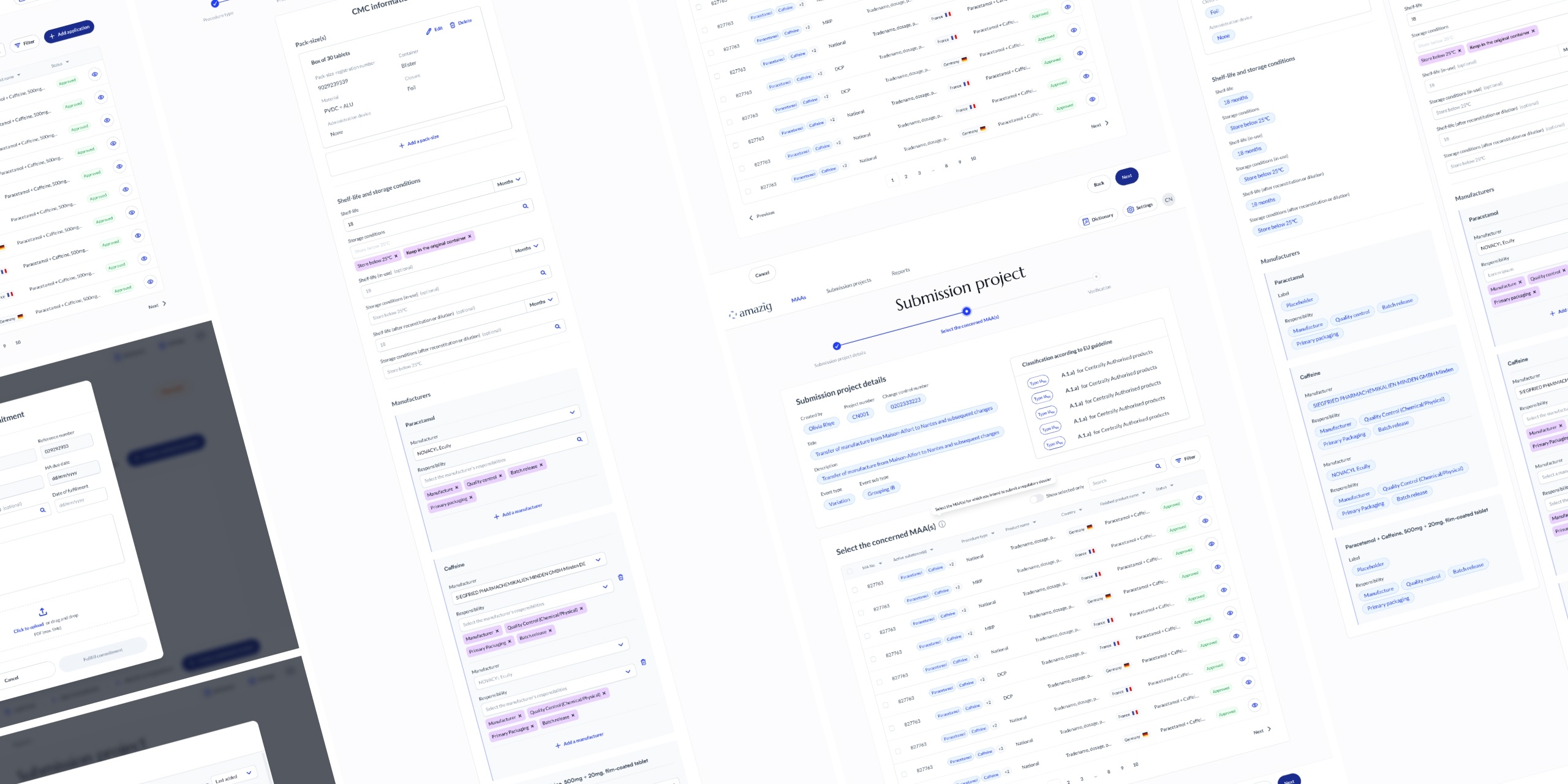
Impact & Next Steps
Delivered a full prototype (70+
screens) covering end-to-end workflows, enabling investor and
regulatory presentations. Set the foundation for MVP development and
validation.
Next: refining into a fully clickable
MVP, testing with pharma teams, and continuous improvements while
development is ongoing.
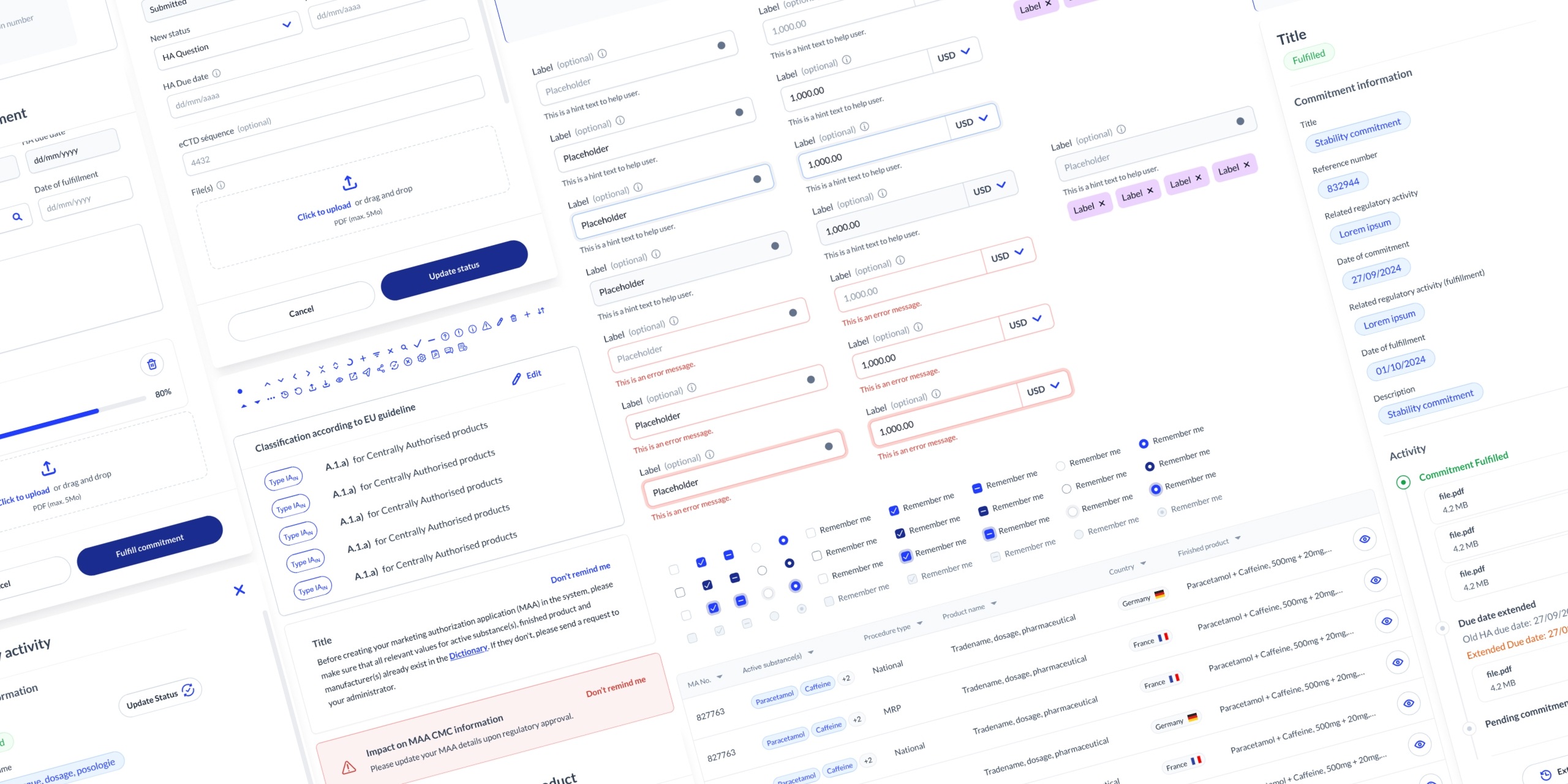
Key Learnings
The importance of balancing compliance
and usability in a highly regulated industry, using early prototypes
to quickly align with developers and stakeholders, and building shared
understanding through a regulatory glossary and a day of expert
shadowing.
CEO Feedback
”I had the opportunity to work with
Adrien on a complex SaaS project. Even though the topic was highly
technical, he quickly understood the challenges and came up with
smart, practical solutions with a lot of agility and responsiveness.
Working together was smooth and enjoyable, and his expertise really
pushed the project forward. His structured approach also made the
developers’ work much easier, which saved us a lot of time. I can only
highly recommend him.” Hina R.
